What Are The Three Main Functions Of The Battery?
The automotive battery has three major functions to perform.
- To supply direct current for starting, lighting, and ignition.
- To control the voltage in the electrical system.
- To supply direct current when the electrical load of the vehicle exceeds the output of the vehicle generator.
The current taken from the battery for starting, lighting, and ignition (called the battery discharge) must be replaced by adequate charging by the vehicle generator. If this is inadequate the battery must be recharged from an outside source.
How Are Lead-Acid Battery Constructed?
A battery consists of a number of cells connected in series. Each cell has a nominal terminal voltage of 2V Three cells in series comprise a 6V battery and six cells in series a 12V battery.
Each cell is fitted with a number of positive and negative plates, consisting of lead alloy grids,
The plate is filled with lead peroxide.
Fig. 6.1. The positive plate of the battery cell.
the positive plates being filled with lead peroxide (Fig. 6.1) and the negative plates with spongy lead (Fig. 6.2).
The plate is filled with spongy lead.
Fig. 6.2. The negative plate of a battery cell.
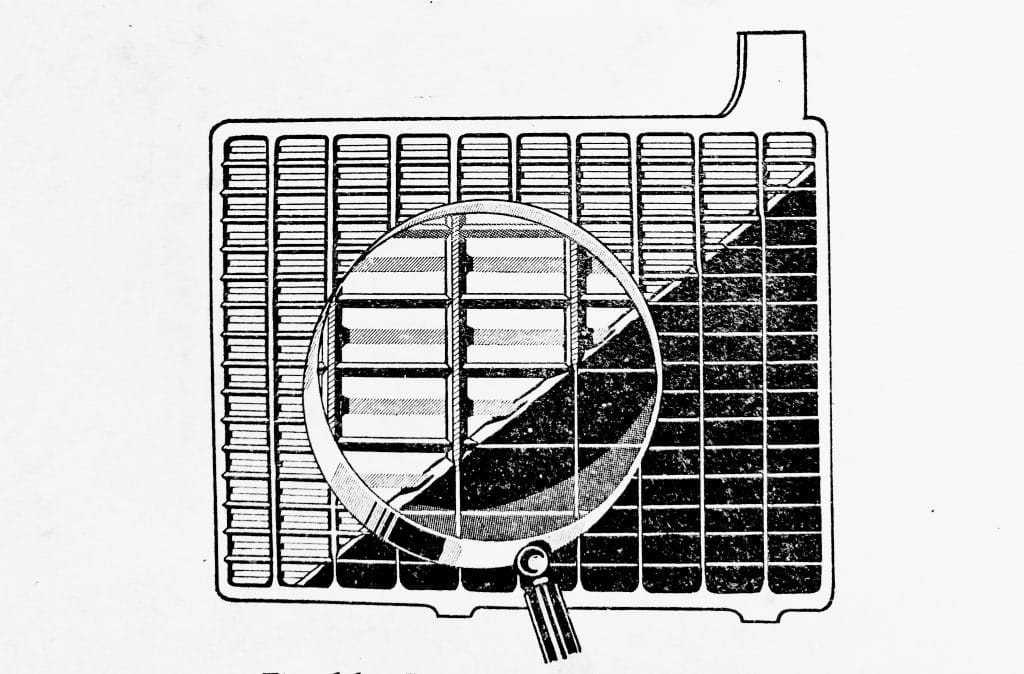
Both the positive and negative plates are welded to post straps and form separate groups which are interleaved as shown in (Fig. 6.3).
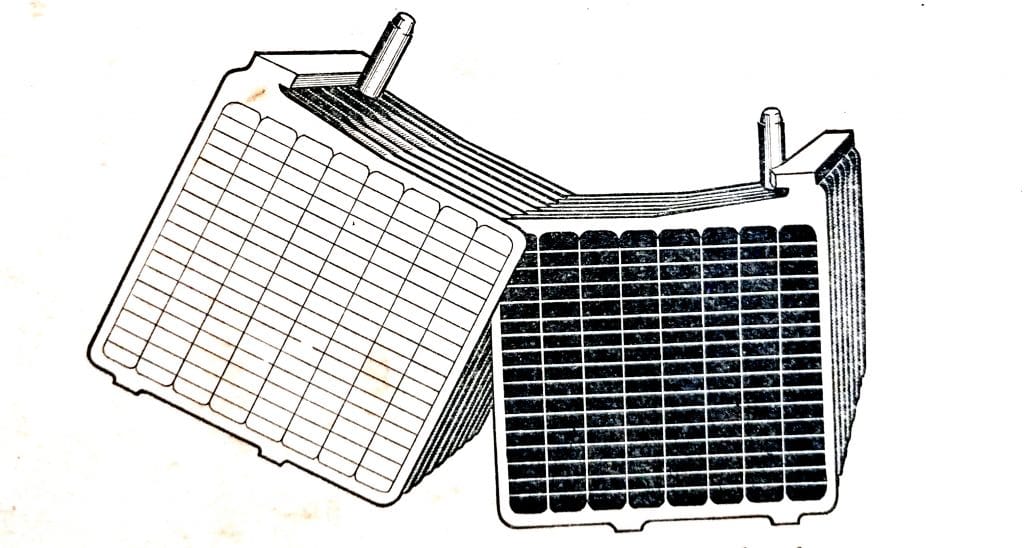
Fig. 6.3. Positive and negative plates interleaved to form a cell element.
Separators made of treated wood, porous rubber, or plastic material are fitted between the plates to prevent their touching. The separators have ribs on the side facing the positive plates to improve operating efficiency. An impression of a wood-type separator is shown in (Fig. 6.4).
The combination of the plates and separators is called an element, as shown in (Fig. 6.5), each group of plates having a post that projects through the cell covers.
Separators may be made of wood, porous rubber or plastic.
Fig. 6.4. One type of separator.
Each complete element is assembled in the familiar battery molded container, the complete assembly being as (Fig. 6.6) and (Fig. 6.7.) The element E is placed in the battery compartment J, is enclosed with the lid L through which the posts protrude, and are connected to the adjoining cell by the strap C.
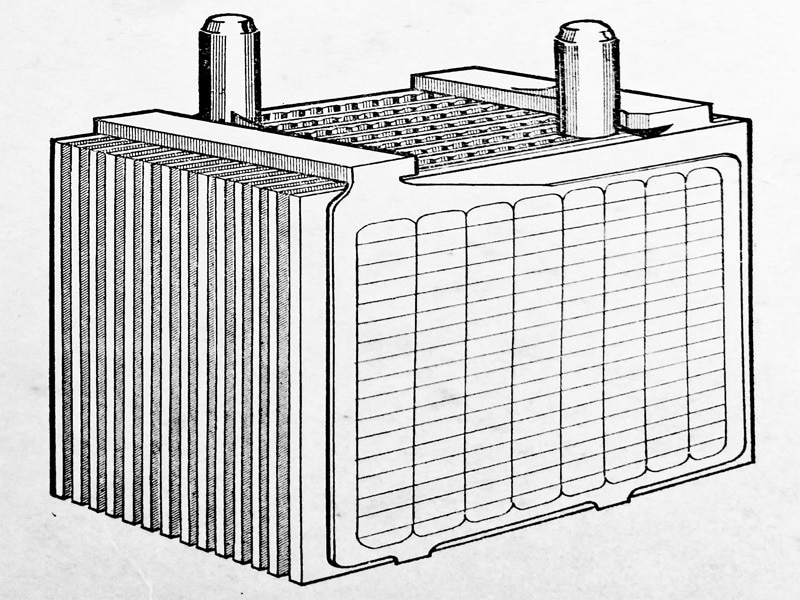
The positive and negative plates are welded to post straps, each group having a post that projects through the cell covers. Separators are fitted between the plates.
Fig. 6.5. Assembled battery cell.
The plug S is used in the vent opening in the cover and removed for topping up. The battery posts are tapered, the positive post being slightly larger than the negative.
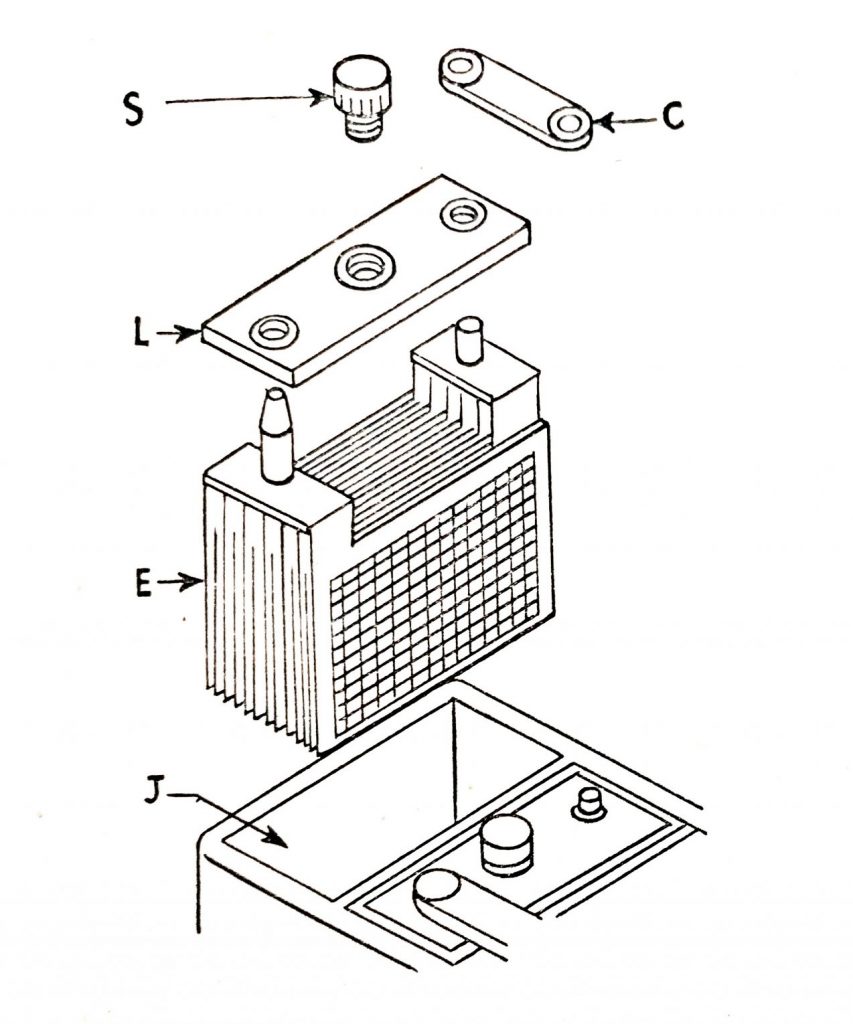
S. Cell vent plug.
C. Strap.
L. Lid.
E. Element.
J. Battery compartment.
Fig. 6.6. Exploded view of the lead-acid cell.
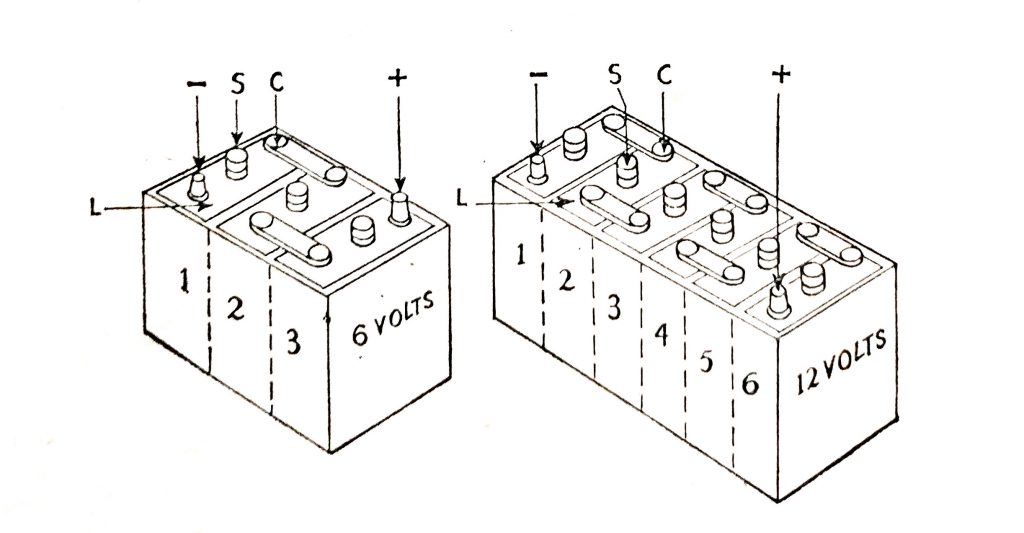
Each cell is 2V and it will be seen that the 6V battery comprises three cells and the 12V battery is made up of six cells. The positive and negative battery terminals are shown.
Fig. 6.7. 6V and 12V battery assemblies.
How does a Lead-Acid Battery discharge?
The active material of the positive plates of a lead-acid battery cell is lead peroxide and of the negative plates, spongy lead. The strength of the electrolyte is at its maximum and the cell voltage will be about 2V.
When an electrical load is connected to the battery and the current taken from it, the battery becomes discharged. Current flows from the cell positive terminal to the electric load to the negative terminal. Flowing via the plates and battery electrolyte to the positive again and forming a closed electrical circuit. The flow of current inside and outside the battery is shown in (Fig. 6.8.)
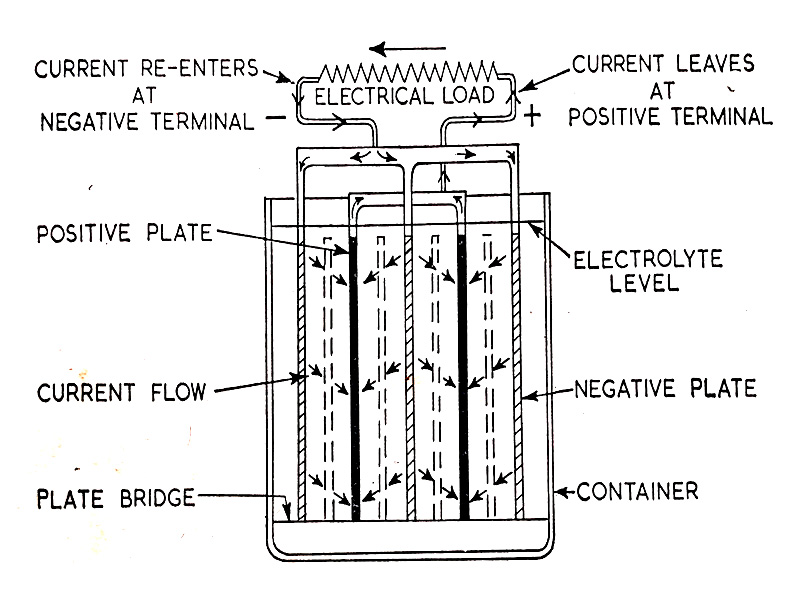
Fig. 6.8. The flow of current in the battery cell during discharge.
The flow of discharge current causes a chemical change to take place within the cell, as shown in (Fig. 6.9.) Some of the sulfate in the sulphuric acid in the electrolyte leaves the electrolyte and combines with the lead peroxide on the positive plates, and the spongy lead on the negative plates, to change both materials to lead sulfate. Oxygen leaves the positive plates and combines with hydrogen in the electrolyte to form water, thus weakening the electrolyte. As the discharge continues, lead sulfate accumulates in both the positive and negative plates and the electrolyte becomes continuously weaker. This progress continues until a condition is reached which makes it impossible any longer for the battery to produce the required flow of current when it is said to be “ discharged”.
Battery Charging-What Happens When a Battery Is Fully Discharged?
When the battery is discharged, direct current must be sent through it, either from the vehicle generator or from an outside charging source.
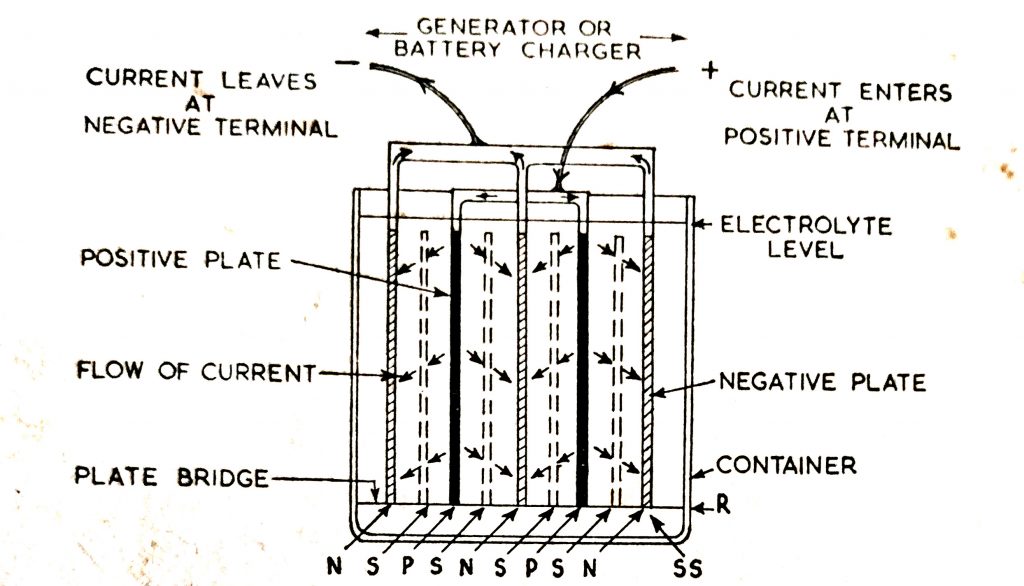
As shown in (Fig. 6.10), direct current enters the battery at the positive terminal, flows from the positive plates through the electrolyte to the negative plates to the negative terminal.
Discharging
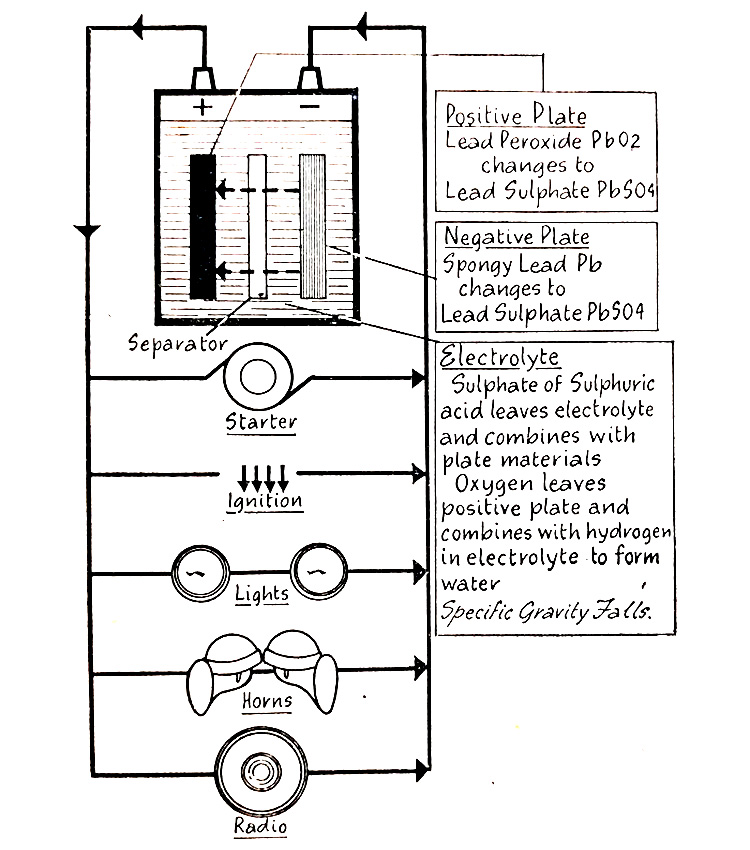
Fig. 6.9. Showing the chemical change occurring within the cell during discharge.
The flow of current inside the battery is in the reverse direction to that during discharge.
Fig. 6.11 shows the chemical reactions which take place during charge which are the reverse of those occurring during discharge. The sulfate returns from the positive and negative plates to the electrolyte and the oxygen in the electrolyte return to the positive plates. The lead sulfate of the positive plates again becomes lead peroxide and the lead sulfate of the negative plates, spongy lead. The increase of sulfate and the return of oxygen to the positive plates strengthens the electrolyte and the cell voltage rises.
The current flow inside the battery is in the reverse direction to that during discharge.
Fig. 6.10. The flow of current in a cell during charge.
How Is Specific Gravity Determined?
The changes which take place in the strength of the electrolyte are shown by its specific gravity. Specific gravity is defined as the weight of a volume of liquid divided by the weight of the same volume of water at the same temperature.
Sulphuric acid is heavier than water and thus any mixture of the acid with water will weigh more than the same volume of pure water. So with sulphuric acid in the electrolyte it is heavier than water and, if the battery is fully charged, the electrolyte weight will be 1.280 times that of an equal volume of water and is described as having a specific gravity of 1.280. When the battery has discharged the weight of the electrolyte will be less, about 1.150 times the weight of an equal volume of water, i.e., a specific gravity of 1.150.
What Is the Most Accurate Way to Measure Specific Gravity with a Hydrometer?
The specific gravity gives a reasonable idea of the state of charge of a battery. This is done by using a hydrometer as shown in (Fig. 6.12.) The hydrometer consists of a glass barrel and syringe for sucking a sample of the electrolyte into the barrel in which there is a glass hydrometer floating with an inside scale marked in units of specific gravity.
The hydrometer floats high in the electrolyte (as shown on the left of the diagram). When the specific gravity of the electrolyte is high and low (as on the right of the diagram) when the specific gravity is low. The position of the hydrometer float and the readings of specific gravity shown in (Fig. 6.12) relates to a charged and discharged automobile lead-acid battery.
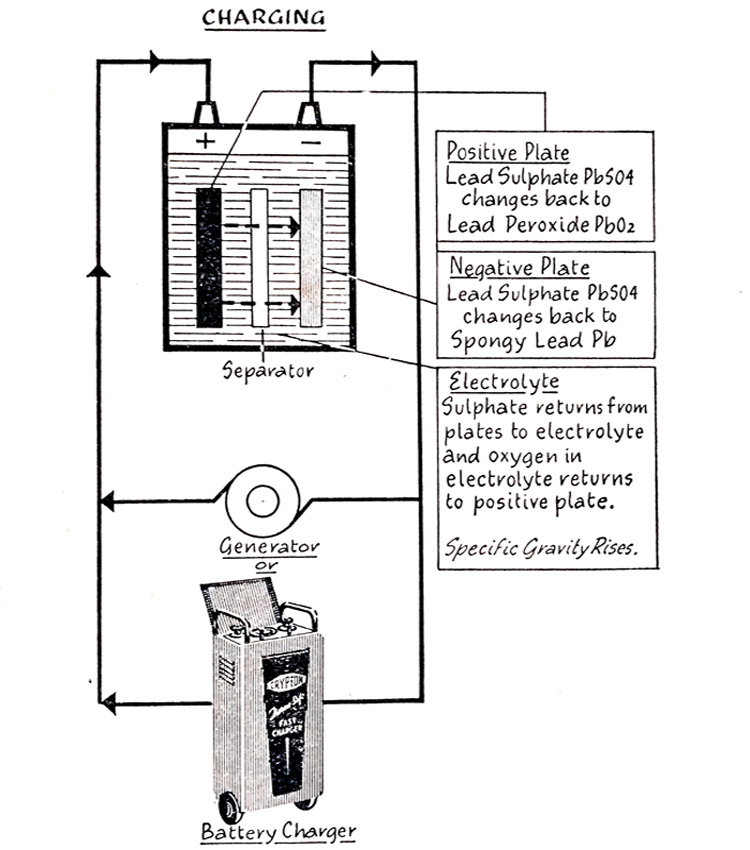
Fig. 6.11. The chemical reactions in a cell take place during charging.
Fig. 6.13 shows diagrammatically the relationship between specific gravity and the state of charge of the battery. What is measured is the amount of acid which remains available in the electrolyte as shown by the shading of the diagram. At full charge, all the available acid is in the electrolyte and the specific gravity is therefore high and of the order of 1.270 to 1.285. As the battery is discharged acid leaves the electrolyte and combines with the active materials on the battery plates and the specific gravity falls. As discharge continues the acid becomes weaker and the specific gravity falls until the battery reaches a discharged condition of about 1.150.
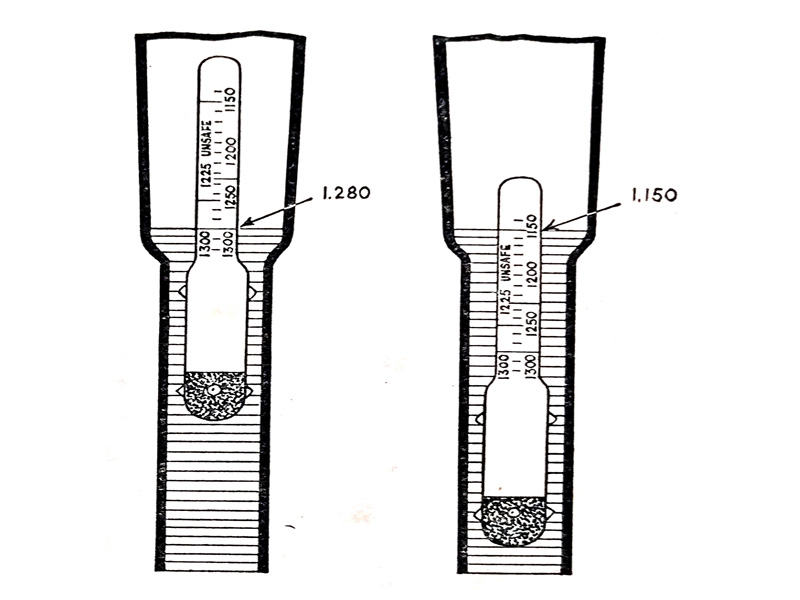
- (left) Position of hydrometer float and reading for a fully-charged battery.
- (right) Position of hydrometer float and reading for a discharged battery.
Fig. 6.12. Principle of the hydrometer.
A hydrometer test will give an approximate indication of the state of charge of the battery cells. When making the test, the level of the electrolyte in the cells must be of normal height and thoroughly mixed with any water that may be added. The hydrometer should be held so that the acid in the float is at eye level as shown in Fig. 6.14.
At a temperature of 60°F the average figures for specific gravity are as follows:
| Specific Gravity | State of Charge (percent) |
| 1.280 1.250 1.220 1.190 1.160 1.130 | 100 47 50 25 Little useful energy left Fully discharged. |
Hydrometer readings are correct only at one temperature; at temperatures other than that for which a hydrometer is designed, corrections are needed. The standard hydrometer temperature is 60°F and test readings must be corrected for different temperatures by adding
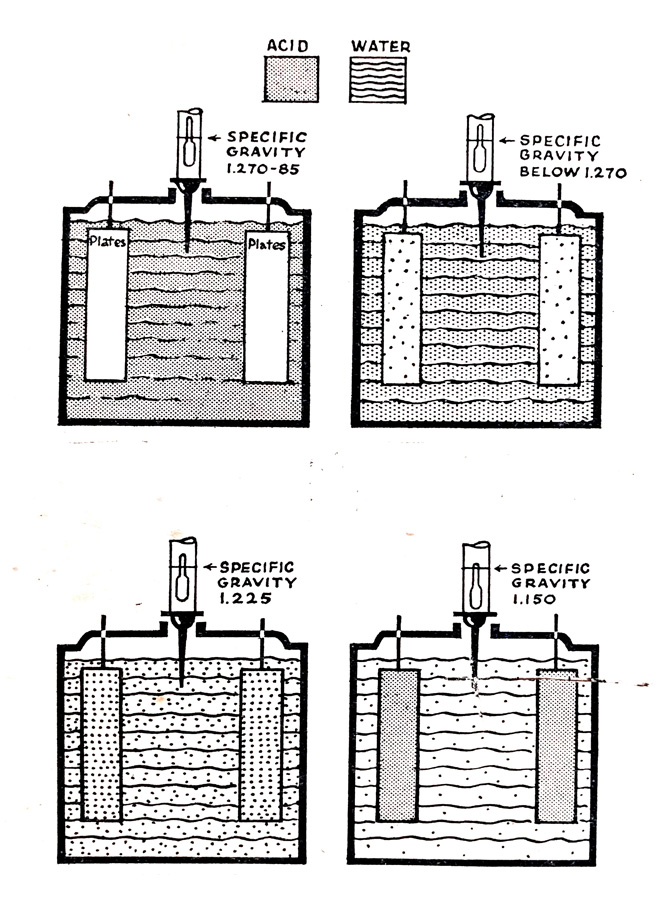
Fig. 6.13. Relationship between specific gravity and state of charge.
four points (0.004) of specific gravity for each 10°F above 60°F, and deducting four points for each 10°F below 60°F. At ordinary temperatures, the effect on hydrometer readings is too small to necessitate corrections but at extremes of temperature, corrections must be made. This is evidenced by the following examples.
Example 1.
A hydrometer reading of 1.265 normally indicates that the battery is about 90 percent charged.
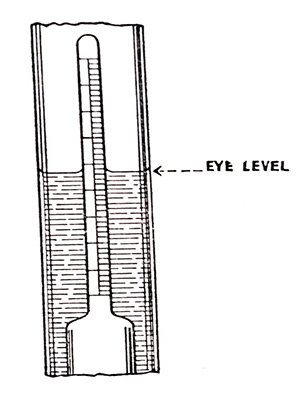
Fig. 6.14. The hydrometer should be held so that the acid in the float is at eye level.
If, however, the electrolyte temperature says, only 20°F, i.e., 40° below the standard hydrometer temperature, the reading must be reduced by four points for each 10°F below 60°F, i.e., by 4 X 4 = 16 points. The corrected reading now becomes 1.265 – 0.016 = 1.249, showing that the battery is only about 75 percent charged.
Example 2.
A hydrometer reading of 1.250 would normally indicate about a 75 percent charge. If, however, the electrolyte temperature is high, say, 120°F, i.e, 60°F above the standard temperature,
the reading must be increased by 4 x 6 = 24 points,
giving a corrected reading of 1.250 + 0.024 = 1.274,showing the battery is about 90 percent charged.
In the first example concerning cold-weather testing, a battery only 75 percent charged might easily have been passed as satisfactory and subsequently fitted to the vehicle. In cold weather, a low state of charge would cause probable starting trouble and might result in the battery being returned with a complaint.
In the second example, a temperature of 120°F is experienced at the end of a fast battery charge. If the hydrometer reading is not corrected for temperature it would be assumed that the battery is only 75 percent charged and needs further charging, whereas it is about 90 percent charged and quite suitable for service.
When making hydrometer tests, specific gravity readings should not be taken immediately after batteries are discharged at a high rate such as prolonged engine cranking. This type of heavy current discharge weakens the acid near the battery plates and the readings become unreliable until the weak acid has had time to get thoroughly mixed with the remainder.
What Is the Open-Circuit Voltage of the Battery?
The open-circuit voltage of fully-charged battery cells of all sizes and with the acid of 1.280 specific gravity is approximately 2V. The voltage of the cell will fall when a load is connected to it, the rate of fall varying with the size of the cell, its state of charge at the beginning of the discharge, the electrolyte temperature, and the design and condition of the battery. With a light load, the fall in voltage will be gradual as shown in Fig. 6.15, which shows the voltage fall of a 60-ampere-hour battery with a load drawing of about 6A.
A heavy load connected to the battery, such as turning the starter to crank the engine, will cause a much steeper voltage fall, to about 1.5V per cell. If continued efforts are made to start a difficult engine the voltage will rapidly fall still lower. The condition is shown in Fig. 6.16 where the effects of repeated attempts to start the engine are shown by a rapid fall in voltage which soon becomes zero. In practice, a battery will give many more starting efforts than the five illustrated but the illustration gives a correct impression of the effect of repeated operations of the starter motor.
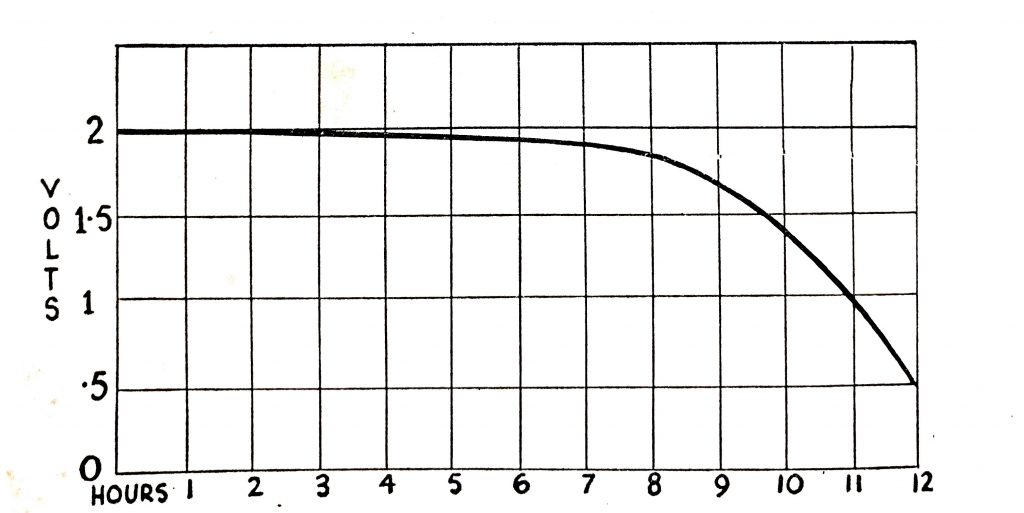
When a load is connected to a fully-charged cell the voltage will fall. The illustration shows the amount of fall with a light load (6A) for a 60-ampere-hour battery.
Fig. 6.15. Cell voltage on normal load.
If a battery cell is tested for specific gravity and voltage when on an open circuit, i.e., with no current flowing into or being taken from it, it will be found that the open-circuit voltage of the battery will vary as well as its specific gravity according to the state of charge of the cell.
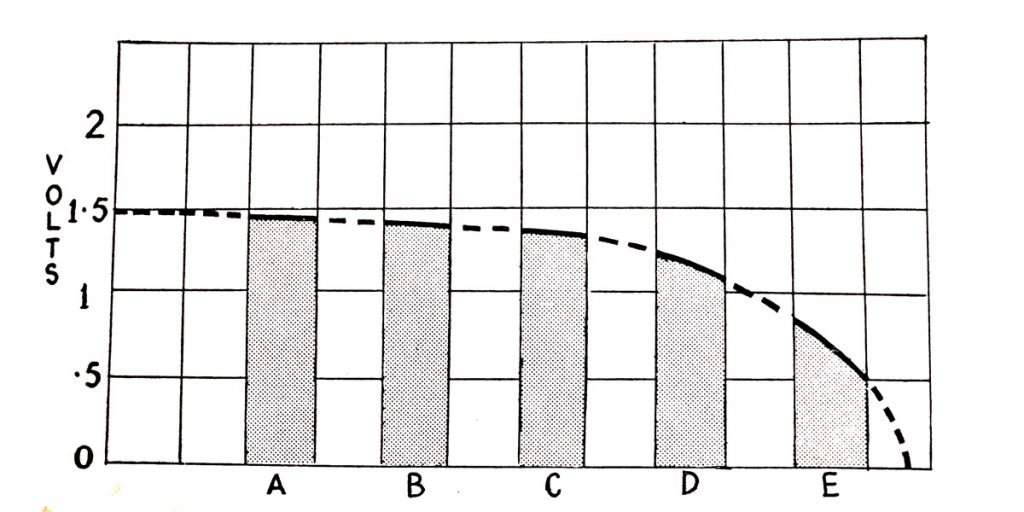
The illustration shows the heavy fall of voltage in a cell when repeated attempts are made to start the engine.
Fig. 6.16. Cell voltage on heavy load for starting.
The approximate figures are as follows.
| Specific Gravity | Cell Open circuit Voltage | The approximate State of Charpercentcent) |
| 1.280 1.250 1.220 1.190 1.160 1.130 | 2.12 2.09 2.065 2.04 2.01 1.98 | 100 75 50 25 Commercially discharged Fully discharged. |
It will be seen that the variation in cell open-circuit voltage is very small, only a few hundredths of a volt. The variation is, however, in direct proportion to the specific gravity and provided a voltmeter is available which will read accurately within limits of one-hundredth volt, the battery can be reliably tested for the state of charge by measuring the cell voltage instead of testing the specific gravity. Because of the greater simplicity and speed of testing, special voltmeters have been designed, capable of reading within the required limits of accuracy and these have become very popular as a replacement for the hydrometer.
How Do You Check Open Circuit Voltage with a Voltmeter?
A modern type of open-circuit voltage tester consists of a moving coil voltmeter with a special expanded scale, calibrated 0 to 40 points, each point equal to one-hundredth of a volt and approximately ten points of specific gravity. The instrument is zoned so that all cells which are more than percent charged give a reading in an area of the meter scale marked “Serviceable ” and cells less than percent charged in an area marked “ Recharge “.
The test prods of the voltmeter are connected across each battery cell in turn, when the position of the meter needle will indicate the state of charge of the cell; the uniformity of the cell readings will show the condition of the battery. All cells should show a reading in the “ Serviceable” zone and be uniform within six points. If the cell readings are not uniform within six points the battery is possibly defective and should then be recharged and retested.
The open-circuit voltage tester, when used in accordance with the instructions supplied with the instrument, will give a reliable indication of the state of charge of a battery in the vehicle and give a guide as to its condition. It must always be used when the battery is on an open circuit with no current flowing into or out of the battery. It cannot be used to test batteries whilst on charge or discharged.
When testing batteries that have been on charge there will be an abnormally high voltage and this must be dissipated by turning on headlamps, radio, and accessories. When testing a battery of a vehicle just bought off the road, the headlamps should be turned on for about one minute, then turned off and tests made after waiting two or three minutes. Where batteries have been discharged immediately before testing they will have an abnormally low voltage which will gradually become restored if the battery is allowed to stand. Therefore, when making an open-circuit voltage test the battery should be allowed to stand for some time before the test is made.
How Do You Measure Battery Capacity?
The term ” battery capacity” refers to the product of the current in amperes that a battery can give and the number of hours during which it can supply this current before the voltage falls below a certain minimum.
The “number of hours” is normally an agreed period (e.g., 10 hours) and must always be stated.
The number of hours is called the “rate ” of discharge. A battery having a capacity of 100Ah at the 10-hour rate will give 10A for 10 hours.
A battery with a capacity of 100Ah at the 5-hour rate will give 20A for 5 hours. The actual capacity of a given battery depends upon the number and size of plates in the cells and the amount of electrolyte present. The starting capacity of a battery is in proportion to the plate area. Since a high starting capacity is needed with automobile batteries the maximum plate area is necessary. This is provided by fitting the battery with a large number of thin plates.
Effect of Temperature on Battery Capacity.
Battery capacity is greatly reduced by cold weather because low temperatures have a numbing effect on the chemical action of the battery and result in a considerable fall in cranking performance A battery with 100 percent cranking power at 80°F gives only 65 percent cranking power at 32°F and only 40 percent at 0°F. This is shown in Fig. 6.17.
Under cold-weather conditions, more power is needed to start the engine. An engine that needs 100 percent cranking power at 80°F will require 165 percent power at 32°F and 250 percent at 0°F. This is shown in Fig. 6.18.
Comparison of Cranking Power Available from Fully Charged Battery at 80°, 32 and 0° F.
Comparison of Power Required to Crank Engine with S. A.E. 20 Oil at 80°, 320 and 0° Fahrenheit.
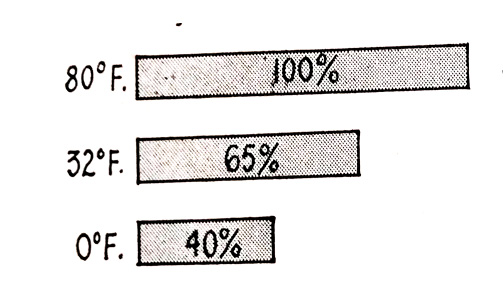
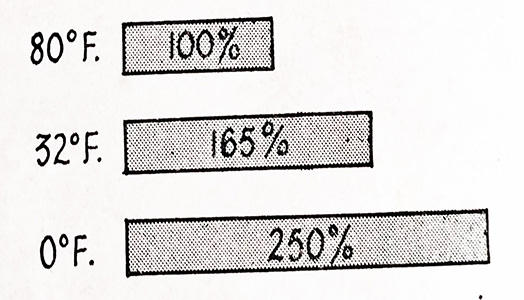
Fig. 6.17. Battery cranking voltages at various temperatures.
Fig. 6.18. Engine power requirements at various temperatures.
If the battery is in a low state of charge its ability to start an engine under low-temperature conditions is seriously impaired. Fig. 6.19 shows, for example, that at a temperature of 32°F a half-charged battery will give only 32 percent of its normal cranking power, as shown in Fig. 6.18, the engine requires 165 percent more power. Thus, we see the great importance of keeping batteries in a condition of full charge, particularly under cold-weather conditions.
Under conditions of extreme cold, there is a possibility that the battery electrolyte may freeze. This is made more likely if the battery is not kept fully charged because, at low states of charge, more of the electrolyte is water and the possibility of freezing is therefore increased.
Where batteries are operating under high-temperature conditions they give a more lively performance than at normal temperatures.
Comparison Showing How the State of Charge Affects Cranking Power
at Temperatures of 80°, 32°. and O°F.
Showing Percentages Compared to Capacity in the Fully Charged State at 80°F. equal to 100%
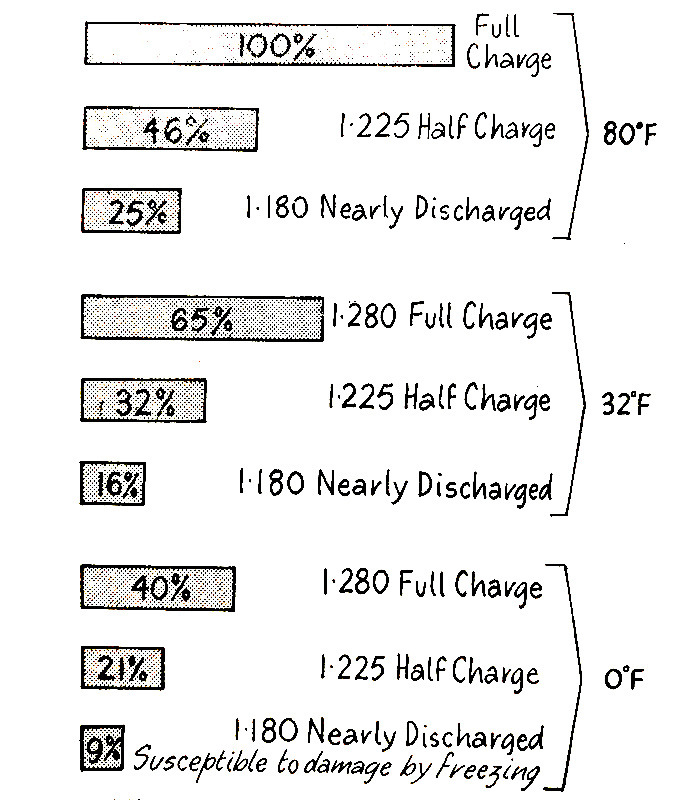
Fig. 6.19. State of charge effect on cranking power at various temperatures.
There is, however, a corresponding disadvantage because, at these higher temperatures, the corrosive property of the sulphuric acid is greater and there is more risk of disintegrating the lead grids and separators. For this reason, battery manufacturers specify lower specific gravities in tropical climates; fully charged specific gravities are of the order of 1.250 to 1.255 instead of the normal figure of 1.280.
How Does a Lead-Acid Battery Self-Discharge?
If lead-acid batteries are left standing in a charged or semi-charged condition they will slowly discharge. This will cause a small loss of battery capacity which will vary according to temperature. At normal temperatures of 60°F to 80°F, the loss over ten days will be about 0.002 points of specific gravity per day.
To limit the effects of self-discharge batteries should be stored in as cool a place as possible, away from radiator or hot-air ducts in winter, and from direct sunlight in summer.
Before fitting stored batteries to the vehicle, a test should always be made to determine the extent of self-discharge. If the electrolyte specific gravity has fallen to 1.240 or less, the battery should be given a boost charge. The best procedure is to give batteries a routine boosting charge at intervals of about thirty days at a charging rate of approximately 1A per positive plate.
Causes of Poor Battery Performance.
Unsatisfactory battery performance can be caused by faulty conditions, either within the battery or in the vehicle’s electrical system. Faulty conditions within the battery are disclosed by cells showing unequal states of charge due to such internal faults as buckled plates, damaged separators, disintegrated active materials, sulphation, etc. External faults in the electrical system result either in an excessive power drain on the battery or insufficient charging; in both cases, a normally healthy battery will show a low state of charge on all cells but will respond readily to charging from an outside source.
The most frequent cause of poor battery performance is age, since the constant charging and discharging of the battery gradually lowers its capacity. A further cause is a failure to provide adequate maintenance, e.g., neglecting to top up the electrolyte periodically, thus allowing the level to sink and exposing the plate surfaces to the atmosphere. This causes hardening and crystallization of the plates and reduces their effective area, thus lowering the battery capacity and imposing a greater strain on the battery, thereby shortening its life. Sometimes the replacement of spilled electrolyte with water may cause the electrolyte to become too weak.
This will result in increased internal resistance and change the relationship between the specific gravity and the state of charge. Hydrometer readings become false and bear little relation to the true cell condition. It sometimes happens that spilled electrolyte is replaced with an acid solution. This causes the specific gravity to become too high, causing battery deterioration and again producing false hydrometer readings. The mounting of batteries in the vehicle sometimes causes trouble and leads to cracked cases, distorted fixing lugs, and excessive current leakage. Overcharging of batteries will cause excessive gassing of the electrolyte and may result in overspill, wet and leaky cell lids, buckled plates, burst lids, and corroded cables and cable connections.
How Do I Stop My Car Battery from Overcharging?
Overcharging means putting more current into a battery than is needed for the full conversion of the active material from a discharged to a charged condition. The effect of overcharging is to decompose the water in the electrolyte into hydrogen gas and oxygen gas. Excessive gassing will develop and the gas bubbles may wash active material away from the plates and will also carry moisture and acid away from the cells as a fine mist.
This will cause the remaining electrolyte to become more concentrated which will tend to char the separators and make the active material of the plates granular. If overcharging continues it will create high internal heat which may soften and distort the container and the sealing compound. The positive plate grids may become corroded and the separators and negative plates damaged.
What Causes Undercharging of a Car Battery?
The operation of a battery in an undercharged condition puts a heavy strain on it because it is unable to develop its full power at the proper voltage. In a seriously undercharged battery, the electrolyte may freeze under severe weather conditions. This is shown in the following table.
Freezing of Electrolyte.
| Specific Gravity | The temperature at which freezing Commences (°F) |
| 1.280 1.120 1.200 1.150 1.100 | -90 -62 -16 +5 +19 |
These temperatures are those where freezing will commence taking place. The electrolyte will not freeze solid until lower temperatures are reached. As the table shows, the only real danger of freezing occurs when the batteries are in a low state of charge. A battery three quarters charged or more is in no danger of freezing.
What Causes Sulfation on a Battery?
The most serious effect of prolonged undercharging is the formation of a hard crystalline type of sulfate in the plates. This increases the battery resistance and, once the sulfate starts to form, the resulting increased resistance reduces the charging rate for a given charging voltage and thereby accelerates the trouble. Dense and hard sulfate sets up strains in the positive plates and causes a distortion called “buckling”. This is accelerated if the sulfated battery is due to sudden overcharging which might occur if a vehicle has been left standing for a long time or has been subject to undercharging and then used for a long journey. Regulators which are out of adjustment can cause a similar condition.
Do Batteries Require Water?
Water is one of the four essential substances used in lead-acid batteries. As a result of normal service, a certain amount of water is lost by evaporation and must be replaced. This should be done as soon as the level of the electrolyte in the cells falls to the top of the separators. Failure to do this will expose the tops of the plates which may become permanently sulfated. The electrolyte will also become too strong and may char and disintegrate the wood separators.
Distilled water should always be used for replacing water loss through evaporation. Sulphuric acid should never be added to a battery unless it has been knowingly lost and should then be replaced strictly under conditions issued by the battery and acid manufacturers.